Biomediated
precipitation of calcium carbonate metastable phases in hypogean environments:
a short review
S. SANCHEZ-MORAL
Museo Nacional de Ciencias Naturales, CSIC, José Gutiérrez Abascal 2, 28006
Madrid, Spain
J.C. CAÑAVERAS
Departamento de Ciencias de la Tierra y del Medio
Ambiente, Universidad de Alicante, Campus San Vicente del Raspeig, 03080
Alicante, Spain
L. LAIZ
C. SAIZ-JIMENEZ
Instituto de Recursos Naturales y Agrobiologia, CSIC, Apartado 1052, 41080
Sevilla, Spain
J.BEDOYA
L. LUQUE
Museo Nacional de Ciencias Naturales, CSIC, José Gutiérrez Abascal 2, 28006
Madrid, Spain
Abstract..
Natural precipitates
of metastable polymorphs of CaCO3, such as vaterite, are rarely
found in nature, however they have been widely synthetised in laboratory under
particular conditions (i.e. supersaturated solutions, relative high
temperatures, etc.). By SEM and XRD we recognize vaterite spherulites from
culturable microbial colonies isolated from hypogean environments. Spherical
bodies (~10 m in diameter), probably composed of vaterite, occur in
submilimetric microbial mats and biofilms on volcanic substrates (Saint
Callixtus Catacombs, Rome, Italy) and karstic caves (Altamira, Candamo and Tito
Bustillo caves, Spain, and Grotta dei Cervi, Italy) where cyanobacteria and
actinomycetes are the major microbial components. These particles form beneath dense biofilms, where particular
physicochemical conditions are developed by the microbial activity. Natural
biofilms seems to generate microenvironments favoring the formation and
preservation of metastable CaCO3 polymorphs. This also shows a major
role of microbes in processes of low-temperature alteration of different
hypogean rock-substrates
Keywords: vaterite, monohydrocalcite, calcite, actinomycetes,
karstic cave, catacombs
The
research was supported by the European Commission, contract EVK4-CT2000-00028.
This is part of the UNESCO/IUGS IGCP 448: “World Correlation on Karst
Ecosystem”. We thank R. González for their excellent technical assistance. Many
thanks are extended to Editor Ulrike Brehm and the two anonymous reviewers for
their assistance and useful comments on the draft manuscript.
Address correspondence to Prof. J.C. Cañaveras, Dept. Ciencias de la Tierra y del Medio Ambiente,
Universidad de Alicante, Ap. 99, 03080 Alicante, Spain. E-mail: jc.canaveras@ua.es
Microorganisms, particularly bacteria, inhabit
all possible environments of the biosphere including subterranean ones, playing
a role in geological processes such as mineral growth and dissolution, and
influencing biogeochemical cycles (Krumbein, 1983; Ehrlich, 1998). In
comparison with other environments, there are relatively few geomicrobiological
studies dealing with hypogean environments (Northup et al., 1997; Northup and
Lavoie, 2001). Microbes, are often harmful for cultural assets (e.g.
Paleolithic paintings), because they are related to constructive (mineral
precipitation) and destructive (substrate dissolution) processes affecting
different substrates (host-rock, speleothems, paintings, etc.) (Monte and
Ferrari, 1993; Saiz-Jimenez, 1995; Dornieden et al., 2000; Cañaveras et al.,
2001). Therefore geomicrobiological studies are necessary to establish the role
that microorganisms play in the microbial-mineral interactions that occur in
hypogean environments, and to elaborate management tools for the conservation
of show caves and tombs.
Laboratory experiments
demonstrate the ability of bacteria isolated from caves to precipitate and
dissolve carbonates (Danielli and Edington, 1983; Groth et al., 2001). However,
the existence or recognition of the metastable phases in hypogean environments
is rare and their organic or bioinduced origin is not always clear.
In the last years, our group has carried out
multidisciplinary studies (geology, microbiology, hydrochemistry, microclimate)
in different caves located in Spain, such as Altamira (Cañaveras et al., 1999;
Sánchez-Moral et al., 1999), Tito Bustillo (Sánchez Moral et al., 1997; Cañaveras
et al., 2001), Candamo (Hoyos et al., 1998), and Grotta dei Cervi, Italy (Groth
et al., 2001). At present, we are carrying out similar studies in a different
hypogean environment, the catacombs of Domitilla and Saint Callixtus in Rome,
Italy. Microbial communities and evidences of microbial activity have been
found in or on the different substrates sampled on Roman hypogea. This work
documents the formation of metastable calcium carbonate mineral phases as an
example of microbial-mineral interactions.
Materials and methods
Site
The four caves studied (Altamira, Tito
Bustillo, Candamo and Grotta dei Cervi) are world-famous for having a
remarkable collection of paleolithic and neolithic paintings and engravings.
From a geologic point of view, all these caves are located in the unsaturated
water zone of karstic systems where processes of dissolution/destruction of
carbonate substrates (mainly limestone) are more important than
precipitation/lithochemical reconstruction processes. The tunnels that constitute
both Domitilla and Saint Callixtus catacombs were dug through the relatively
soft volcanic sediments of Roma. These deposits are tuffs and the so-called
pozzuolana (scoriae in an ash matrix) of alkaline and potash composition.
Building materials are coating part of the hypogean surface, mostly with a
decorative purpose. These materials are usually a mixture of rock fragments in
a carbonated matrix forming a mortar and a thin layer of slaked lime or plaster
over which some paints were made. These cover some cubicles, and crypts.
Mineralogy (XRD), chemical composition (A.A.S.,
ICP, EDS), porosity (Hg porosimetry)
and petrographic features (SEM and transmitted light microscopy) of hypogean
substrata have been characterized. Special attention was given to
biofilm/substrate interfaces. Particular
care was taken during sample preparation for SEM. Most of the samples were
fixed in situ with glutaraldehyde and
later, in the laboratory, were gradually dehydrated and dried to prevent the formation
of artifacts.
Hydrochemical
characterization of hypogean waters (dripping, condensation, etc.) were carried
out. Temperature, electrical
conductivity and pH of water samples were measured in situ, and CO2, HCO3- and CO32-
contents were analyzed using standard titration methods. Further, chemical
analyses were performed in the laboratory using a Perkin-Elmer atomic
absorption spectrometer or by an
ionic capillary electrophoresis technique with a QUANTA 4000 capillary
electrophoresis instrument. Chemical
speciations and geochemical calculations were obtained by means of the computer
program PHRQPITZ (version 0.2, 1990), which is a modification of the original
version from Plummer et al. (1988).
Microenvironmental data
(temperature, CO2 concentration, relative humidity, 222Rn
concentration) were obtained from automatic in
situ monitoring systems installed in the studied sites (Sánchez-Moral et
al., 1999). The climatic data of the cave atmosphere were used in geochemical
calculations. The results obtained over annual cycles in different caves showed
that the microclimate conditions were stable and similar in relative air
humidity, with some differences in temperature and carbon dioxide
concentrations. The relative humidity values are always close to saturation
point (above 95%). The mean air temperature in Grotta dei Cervi (about 18°C) is
higher than that measured in Spanish caves (12.5-14.5°C), and the mean annual
air CO2 concentration is smaller than in the case of the Spanish
caves. In order to study the microenvironment of the catacombs, a microclimate
monitoring system, measuring the temperature (16-17°C), relative humidity
(>95%) and CO2 concentrations (1500-3500 ppm) was operating
during 2001 for one day each in Domitilla (5 March) and Saint Callixtus (6
March). Since March 2002
a microclimate monitoring system, measuring lighting time, air/rock
temperature, air relative humidity, CO2 and 222Rn
concentrations was installed in Saint Callixtus. One sensor was installed specifically in the Oceanus cubiculum for
detecting temperature differences between the air and rock surface. The
automatic recording system is programmed to store record every 20 minutes.
First results recorded show that the temperature of the rock surface during the
studied period (March-May) is always slightly higher than the air temperature
ranging from 0.05 to 0.2°C. The combined effect of an increase in CO2
concentration, water vapour emission and temperature variations induced by
visitors can directly affect the intensity and even the development of
bio-colonization. Biogeochemical
cycles must be influenced by the continuous availability of water originated by means of induced
condensation processes.
Sampling and sample location
Sampling have been carried out
in Altamira, Candamo, Tito Bustillo and Grotta dei Cervi caves since 1997 and
in Roman Catacombs (Domitilla and Saint Callixtus) since 2000 to investigate
the microbial population and their interaction with hypogean substrate. Active
stalactites, wall concretions, microbial mats and dripping waters from
different areas were collected aseptically in sterile tubes and kept at 4°C for
two days until microbiological analyses were carried out.
Isolation and identification
Samples were added to a saline solution (0.9%
NaCl), serially diluted and spread on different culture media. The media were
selected taking into account that actinomycetes were the most abundant
microorganisms in these caves (Groth et al. 1999; Laiz et al. 2000; Groth et
al. 2001) and that their colonies, distributed all over the caves, were
partially visible to the naked eye. Tryptone soy agar (TSA), malt-yeast
extract-agar, starch-casein-agar, glycerol-asparagin-agar and water-agar were
used as culture media. The composition of the media is described elsewhere (Laiz
et al. 1999; Groth et al. 2001). The plates were incubated at 28°C for at least
48 h, and up to 8 weeks to record fast and slowly growing bacteria
respectively.
Characterization of the isolates
The isolates were
classified by morphological and chemotaxonomic methods. Carbon utilisation (95
carbon sources) was tested using the Biolog-identification system, but only in
a few cases was identification at the species level possible on the basis of
the Biolog database.
Total cellular
fatty acid methyl esters (FAME) were analyzed using the MIDI system in
accordance with the protocols for cultures grown on solid medium and instrument
specifications recommended by Microbial Identification System, Inc. Delaware,
USA. Comparison with the Sherlock Standard Aerobe database allowed automatic
identification of bacteria. This method was used for identification of bacteria
other than actinomycetes. The identification of streptomycete isolates was
performed using morphological and physiological methods as recommended by
Shirling and Gottlieb (1966). Methods for chemotaxonomic analyses are described
elsewhere (Groth et al. 1999).
Precipitation of salts
The isolates
were tested for salt precipitation using B-4 medium composed of 2.5 g calcium
acetate, 4 g yeast extract, 15 g agar in 1 L distilled water, pH 8 (Boquet et
al., 1973). Precipitation was also tested in liquid medium for the isolates
with higher precipitation capacity in order to collect the crystals for further
analysis. SEM and EDX, X-ray diffraction and FT-IR were routinely used for this
propose.
Crystal analysis
Three Acinetobacter sp. strains were isolated
from Altamira cave. One isolate grew very fast and produced a large amount of
crystals surrounding the colonies and visible after 24 h. After one week, the
crystals were distributed all over the plate. This isolate was only able to use
acetate and pyruvate but not citrate or glucose; the other two strains were
able to used citrate.
In solid
(agar) media structural and morphological analyses were performed on crystals
collected from the colonies surface of a 20-day culture of the most active Acinetobacter isolate. In a similar way,
crystals were obtained in the longest incubated plates from a Rhodococcus isolate. Calcium carbonate
polymorphs were identified by FT-IR spectroscopy (Falini et al. 1996), SEM and
X-ray diffraction. The mineral composition of the precipitates was
characterized by XRD and EDS.
Results and discussion
Grouped or isolated vaterite hemispherical
aggregates precipitated in laboratory cultures of Acinetobacter spp. when B-4 medium with calcium and/or magnesium
acetate was used. Vaterite spherulites ranged from 5 to 20 mm in diameter and showed a radial internal structure (Figure 1A). Some
of the spherulites were hollow (Figure 1B). The production of crystals only
appeared when the Ca acetate/Mg acetate ratio was higher than 1, indicating the
inhibiting role of magnesium in the precipitation of vaterite. When this ratio
was lower than 1, no CaCO3 crystals are produced. The inhibitory
effect of Mg ions on carbonate precipitation by bacteria was also observed by
Rivadeneyra et al. (1985). In Grotta dei Cervi samples, hemispheroidal vaterite
was also obtained from Rhodococcus
isolates (Groth et al., 2001). Similar hemispherical morphologies in CaCO3
precipitates were obtained by Rivadeneyra et al. (1998) from soil bacteria.
They suggested that the spherulites may be the end stage of a progressive
calcification process. XRD analysis indicated that Acinetobacter spp. isolated from Altamira cave and Rhodococcus sp. from Grotta dei Cervi
produced vaterite and calcite. XRD analysis of powdered samples carried out
several days after collection revealed a decrease in the proportion of vaterite
with respect to calcite in moist samples, indicating the partial transformation
of unstable vaterite into stable calcite.
In solid culture media of Grotta dei Cervi
isolates (Rhodococcus sp.),
spherulitic crystals were also produced, but XRD analysis revealed
monohydrocalcite as the main mineral composition. The spherulites range 30 to
70 mm in diameter and also showed a radial internal structure (Figure 1C).
Vaterite, a kinetically
favored, metastable calcium carbonate polymorph, is rarely found in nature but
it has been identified in hypersaline lakes (Giralt et al., 2001),
fluvio-lacustrine sediments (Ito et al., 1999; Lu et al., 2000); drilling muds
(Friedman and Schultz, 1994), mortars (Signorelli et al., 1996; Gleize et al.,
2000); metamorphic aureoles (McConnell, 1960), urinary calculi (Prien and
Fondel, 1947; Diaz-Espineira et al., 1995), and tissues of some organisms
(Lowestam and Abbot, 1975; Falini et al., 1996; Lenaz and Miletic, 2000). Monohydrocalcite (CaCO3·H2O)
is also a scarce Ca-carbonate mineral. It has been identified in
carbonate-silicate microbialites (Léveillé et al, 2001), carbonate hardgrounds
(Last, 1992), polymetallic mineralizations (Ridkosil et al., 1991), lake
sediments (Bird et al., 1991) and salt efflorescences (Jones et al., 1999 and
references therein). Vaterite
and monohydrocalcite are also rare in hypogean environments, only having been
reported in a few karstic caves as constituents of moonmilk, crusts or
coralloids (Hill and Forti, 1997 and references herein).
Vaterite has been synthesized in the laboratory
from oversaturated aqueous solutions (e.g.: Plummer and Busenberg, 1982; Easton
and Claugher, 1986; Ogino et al., 1987), in organic media (e.g.: Mann et al.,
1988; Pach et al., 1990; Falini 2000), in microbial cultures (Rivadeneyra et
al., 1985; Groth et al, 2001), by electrocrystallization techniques (Gabrielli
et al., 1999; Euvrard et al., 2000) or using gas-liquid-solid reactive
crystallizers (Chen et al., 1997). Most vaterite crystals reported in
laboratory experiment shows a globular or spherical morphology, ranging from a
few microns to more than one hundred microns in diameter. Exceptions to this
spherical morphology were mainly the result of experiments looking for habit
modification or specific morphological changes (e.g. Sims et al., 1995; Dupont
et al. 1997). In nature, vaterite commonly shows spherulitic and radial-fibrous
arrangement (Friedman and Schultz, 1994; Giralt et. al 2001). Laboratory
synthesis of monohydrocalcite crystals from bacterial isolates have also been
reported (Rivadeneyra et al., 1998).
Vaterite is a metastable CaCO3
polymorph. Due to its instability, vaterite irreversibly transform into more
stable polymorphs (Bischoff, 1968; Ogino et al., 1987; Kralj et al., 1997).
Factors influencing the nucleation and crystal growth of vaterite are complex.
Experimental reports have shown that less stable carbonate polymorphs appear
due to addition of impurities such as some inorganic ions (Tsuno et al. 2000)
and organic substances (Kitano and Hood, 1965; Falini et al., 1996; Pach et
al., 1990; Mann et al., 1988) or due to kinetic factors (Ogino et al., 1987;
Gabrielli et al., 1999; Elfil and Roques, 2001). However, experimental data
have also indicated some factors favoring the preferential precipitation of a
stable CaCO3 polymorph; these factors are, for example, organic
substances (e.g. fulvic acid) and inorganic suspended impurities (e.g.
montmorillonites) (Vdocic and Kralj, 2000; Kralj and Vdocic, 2000).
Laboratory experiments have revealed that
vaterite preservation is favored by the presence of organic substances, such as
amino acids or mucopolysaccharides (Manoli and Dalas, 2000). Vaterite
preservation is also favored in phosphorus-enriched medium, because dissolved
phosphorus inhibit the precipitation of calcite and/or aragonite; the stability
of vaterite in the presence of phosphate ions was ascribed to the retardation
of both the dissolution of vaterite and the crystallization of calcite (Katsifaras
and Spanos, 1999).
SEM study of more than one hundred samples from
caves and catacombs studied since 1997, has revealed the existence of microbial assemblages, directly or indirectly able
to mediate a variety of constructive and/or destructive processes resulting in
the formation of distinctive fabrics (Cañaveras et al., 2001).
Constructive fabrics include calcified microbes and bioinduced crystalline
precipitates.
Spheroidal bodies, similar to those synthesized
in laboratory cultures, have been recognized at all the studied sites. In
laboratory experiments, crystal nucleation occurs on a substrate where a
complete sphere could not form resulting in a hemisphere. These spherical
bodies, ~10 mm in diameter, were observed in association with biofilms and bacterial
colonies (Figure 1E). Their uniformity in size and their presence in different
substrates (geological and ecological) seem to indicate a microbial origin.
In the Saint Callixtus catacombs, a good
example of these kind of spherical bodies association has been observed within
a 100-150 mm thick subaerial biofilm, (Figure 1E). The biofilm structure consists
of a 5-10 mm thick surface layer of dense filamentous mucus and a 100-140 mm thick needle mat layer. This mat is composed of aragonite needle-fiber
crystals (up to 5 mm long, 0.75 mm wide) in a loosely packed random arrangement (Figure 1E). The
spherical bodies are located in the lower part of the needle mat, in contact
with the rock substratum, and are commonly associated with beaded filaments. The
spheroids show a 0.5-2 mm
thick coating composed of radially-arranged submicron-sized crystals of CaCO3
that probably precipitated around spherical bacterial colonies (Figure 1F).
Unfortunately, the abundance of these CaCO3 spheroids is insufficient
for discriminating their mineralogy using available methodology. Needle-fiber
mats are probably related to biomineralization processes by actinomycetes
(Cañaveras et al., 1999) or fungi (Callot et al., 1985; Verrecchia and
Verrecchia, 94).
Karstic waters of the studied caves are mainly of Ca2+-HCO3-
and Mg-Ca-HCO3- types. They have relatively high
concentrations of total dissolved species, and the presence of high amounts of
dissolved organic matter, NO3- and NH4+
in these karstic waters is a consequence of infiltrational waters passing
through fertiliser-enriched pastures before reaching the cavity (Sánchez-Moral
et al., 1997; Hoyos et al., 1998; Sánchez-Moral et al., 1999; Cañaveras et al.,
1999). Geochemical estimates indicate that hypogean waters (dripping water,
condensation, etc.) are able to precipitate calcite or aragonite. However, for
the precipitation of metastable Ca-carbonates phases, such as monohydrocalcite
or vaterite, higher supersaturation is needed. As previously mentioned, organic
substances, which can be present in biofilm structures, favor the precipitation
of unstable carbonate phases (Falini et al., 1996; Pach et al., 1990; Mann et
al., 1988).
Hydrochemical and microclimatic data confirm that Pco2
values of waters in and around the microbial mats and spheroidal carbonate
particles are lower than air Pco2. Thus, precipitation of carbonate
species must be related to some process controlling the CO2
degassing of thewater. This phenomenon can only occur in a semi-closed
carbonate system (biofilm) where the CO2 removed from the water
cannot be replenished from the cave atmosphere even though CO2 solution
in water from air is very fast. Recent studies have demonstrated that
heterotrophic bacteria are capable of chemolithoautotrophic assimilation of CO2,
and that the carbon is incorporated into all major cell biopolymers (Buzolyova
and Somov, 1999). Cañaveras et al. (1999) demonstrated the biomediated
precipitation of hydromagnesite in crust and moonmilk deposits in Altamira
cave. As a result, microbial activity is able to control the CO2
content of intergranular water in biofilms, leading to supersaturation in both
calcium and magnesium carbonate minerals. An example of the bacterial
capability for sequestering CO2 in solution is the enzyme carbonic
anhydrase. Carbonic anhydrase, an
enzyme that catalyzes the reversible hydration of CO2, is widely
distributed among phylogenetically and physiologically diverse
prokaryotes. Carbonic anhydrase could be responsible for the consumption of CO2
by heterotrophic bacteria in cave systems. This would indicate a far greater
role for this enzyme in nature than previously recognized (Tripp et al. 2001).
The dependence of bacteria on the presence of CO2 for growth or for
overcoming long lag times is known from long time, and CO2 and HCO3- are required for
growth (Smith and Ferry, 2000).
Conclusions
Several facts encourage us to consider as microbial
induced precipitates some carbonate particles found in hypogean environments in
association with heterotrophic bacteria. They probably consist of metastable
calcium carbonate composition, such as vaterite. (i) Some of the naturally
occurring precipitates on the studied hypogean substrates are essentially
identical to those produced in the laboratory. The similarity between
experimental and natural spherical particles (~ 10 m size
spheroids) can be considered as a demonstration of an active process of
bacterial carbonate precipitation. (ii) Samples collected from these sites were
used to isolate microorganisms able to produce crystal precipitates in
laboratory experiments. Although the vaterite composition of natural spheroids
have not been analytically tested, hydrochemical, microclimatic, petrological
and microbiological data seem to indicate that small quantities of CO2
must be consumed by heterotrophic microorganisms in order to explain the
physico-chemical modifications of intergranular fluids that lead to create
supersaturation conditions suitable for neoformation of metastable minerals
(with the highest solubility). This kind of spherical particle could be a
typical representation of the precipitation of metastable carbonate mineral
phases in hypogean environments.
References
Bird MI, Chivas AR, Radnell CJ, Burton HR. 1991.
Sedimentological and stable-isotope evolution of lakes in the Vestfold Hills,
Antarctica. Palaeogeogr Palaeoclim Palaeoecol 84: 109-130.
Bischoff JL. 1968. Catalysis, inhibition and
the calcite-aragonite problem. II The
vaterite-aragonite transformation. Amer J Sci 266: 80-90.
Boquet E, Bordonat A, Ramos Cormenzana A. 1973. Production of calcite crystals by soil bacteria
is a general phenomenon. Nature 246:527-528.
Buzolyova, L.S., Somov, G.P. 1999. Autotrophic
assimilation of CO2 and C1-compounds by pathogenic
bacteria. Biochemistry (Moscow) 64:1146-1149.
Callot G, Guyon A, Mousain D. 1985. Inter-relations
entre aiguilles de calcite et hyphes mycéliens. Agronomie 5: 209-216.
Cañaveras JC, Hoyos M, Sanchez-Moral S, Sanz-Rubio E,
Bedoya J, Soler V, Groth I, Schumann P, Laiz L, Gonzalez I, Saiz-Jiménez C.
1999. Microbial communities associated with hydromagnesite and needle-fiber
aragonite deposits in a karstic cave (Altamira, Northern Spain). Geomicrobiol J
16:9-25.
Cañaveras JC, Sánchez-Moral S, Soler V, Saiz-Jiménez C.
2001. Microorganisms and microbially induced fabrics in cave
walls. Geomicrobiol J 18: 223-240.
Chen P-Ch, Tai CY, Lee KC. 1997. Morphology and growth
rate of calcium carbonate crystals in a gas-liquid-solid reactive crystallizer.
Chem Engin Sci 52: 4171-4177.
Danielli HM, Edington MA. 1983. Bacterial
calcification in limestone caves. Geomicrobiol J 3:1-16.
Diaz-Espineira M, Escolar E, Bellanato J, Medina JA. 1995. Crystalline composition of equine urinary sabulous deposits. Scan
Microsc 9: 1071-1079.
Dornieden Th, Gorbushina AA, Krumbein WE. 2000.
Biodecay of cultural heritage as a spece/time-related ecological situation- an
evaluation of a series of studies. Int Biodeter Biodegrad 46: 261-270
Dupont L, Portemer F, Figlarz M. 1997. Syntehesis and
study of a well crystallized CaCO3 vaterite showing a new habitus. J
Mater Chem 7: 797-800.
Easton AJ, Clauger D. 1986. Variations in a growth
form of synthetic vaterite. Mineral Mag 50: 332-336.
Ehrlich HL. 1998. Geomicrobiology: its significance
for geology. Earth-Sci Rev 45: 45-60.
Elfil H, Roques H. 2001. Role of hydrate phases of
calcium carbonate on the scaling phenomenon. Desalination 137: 177-186.
Euvrard M, Filiatre C, Crausaz E. 2000. A cell to study in situ electrocrystallization of calcium carbonate. J
Cryst Growth 216: 466-474.
Falini G. 2000. Crystallization of calcium carbonates
in biologically inspired collagenous matrices. Int J Inorg Mat 2: 455-461
Falini G, Albeck S, Weiner S, Addadi L. 1996. Control
of aragonite or calcite polymorphism by mollusk shell macromolecules. Science
271:67-69.
Friedman GM, Schultz DJ. 1994. Precipitation of
vaterite (CaCO3) during oil field drilling. Mineral Mag 58:401-408.
Gabrielli C, Maurin G, Poindessous G, Rosset R. 1999.
Nucleation and growth of calcium carbonate by an electrochemical scaling
process. J Cryst Growth 200: 236-250.
Giralt S, Juliá R, Klerkx J. 2001. Microbial biscuits
of vaterite in Lake Issyk-Kul (Republic of Kyrgyzstan). J Sed Res 71: 430-435.
Gleize P, Silva DA, Nappi S. 2000. Ancient rendering
mortars from a Brazilian place. Its characteristics and microstructure. Cement
Concrete Res 30: 1609-1614.
Groth I, Vettermann R, Schuetze B, Schumann P,
Saiz-Jimenez, C. 1999. Actinomycetes in karstic caves of Northern Spain
(Altamira and Tito Bustillo). J Microbiol Meth 36:115-122.
Groth I, Schumann P, Laiz L, Sanchez-Moral S,
Cañaveras JC, Saiz-Jimenez, C. 2001. Geomicrobiological study of the Grotta dei
Cervi, Porto Badisco, Italy. Geomicrobiol. J. 18:241-258.
Ito T, Matsubara S, Miyawaki R 1999. Vaterite after
ikaite in carbonate sediment. Ganko 94: 176-182.
Hill CA, Forti P. 1997. Cave
minerals of the world. Huntsville: National Speleological Society.
Hoyos M, Soler V, Cañaveras JC, Sanchez-Moral, S,
Sanz-Rubio E. 1998. Microclimatic characterization of a karstic cave: human
impact on microenvironmental parameters of a prehistoric rock art cave (Candamo
Cave, northern Spain). Environ Geol 33:231-242.
Jones MS, Wakefield RD, Forsyth G. 1999. The
occurrence of rare minerals on decayed medieval Scottish building sotone
colonised by biological growths. Mat Constr 49 (256): 3-14.
Katsifaras A, Spanos N. 1999. Effect of
inorganic phosphate ions on the spontaneous precipitation of vaterite and on
the transformation of vaterite to calcite. J Cryst Growth, 204:183-190.
Kitano Y, Hood DW. 1965. The influence of
organic material on the polymorphic crystallization of calcium carbonate.
Geochim Cosmochim Acta 29: 29-41.
Kralj D, Brecevic L, Kontrec J. 1997. Vaterite growth and dissolution in
aqueous solution III. Kinetics of transformation. J Cryst Growth 177: 248-257.
Kralj D, Vdovic N. 2000. The influene of some
naturally occurring minerals on the precipitation of calcium carbonate
polymorphs. Wat. Res. 34:179-184.
Krumbein WE. 1983. Microbial Geochemistry.
Blackwell Sci Publ. Oxford. 315 pp.
Last WM. 1992. Petrology of modern carbonate
hardgrounds fron East Basin Lake, a saline maar lake, Southern Australia. Sedim
Geol 81: 215-229.
Laiz L, Groth I, Gonzalez I, Saiz-Jimenez C.
1999. Microbiological study of the dripping waters in Altamira cave (Santillana
del Mar, Spain). J Microbiol Meth 36:129-138.
Laiz L, Groth I, Schumann P, Zezza F, Felske A,
Hermosin B, Saiz-Jimenez C. 2000. Microbiology of the stalactites from Grotta
dei Cervi, Porto Badisco, Italy. Int Microbiol 3:25-30.
Lenaz D, Miletic M. 2000. Vaterite otoliths in
freshwater fishes of the Lower Friuli Plain (NE Italy). Neues Jb Miner Monat
11: 522-528.
Levéillé RJ, Fyfe WS, Longstaffe FJ. 2000.
Geomicrobiology of carbonate-silicate microbilites from Hawaiian basaltic sea
caves. Chem Geol 169: 339-355.
Lowestam HA, Abbot DP. 1975. Vaterite: a
mineralization product of the hard tissues of a marine organism (Ascidiacea).
Science 188: 363-365.
Lu G, Zheng C, Donahoe RJ, Berry Lyons W. 2000.
Controllong processes in a CaCO3 precipitating stream in Huanglong Natural
Scenic District,Sichuan, China. J Hydrol 230: 34-54.
Mann S, Heywood BR, Rajam S, Birchall JD. 1988.
Controlled crystallization of CaCO3 under stearic acid monolayers.
Nature 334:692-695.
Manoli F, Dalas E. 2000. Spontaneous precipitation of
calcium carbonate in the presence of chondroitin sulfate. J Cryst Growth 217:
416-421.
McConnell JDC. 1960. Vaterite from Ballycraigy,Larne,
Northern Ireland Mineral Mag 32: 535-544.
Monte M, Ferrari R. 1993. Biodeterioration in subterranean environments.
Aerobiologia 9:141-148.
Northup DE, Lavoie KH. 2001. Geomicrobiology of caves:
a review. Geomicrobiol J 18: 199-222.
Northup DE, Reysenbach A, Pace N. 1997. Microorganisms
and speleothems. In Hill C, Forti P (eds): Cave Minerals of the World. NSS,
Huntsville: 261-266.
Ogino T, Suzuki T, Sawada R. 1987. The
formation and transformation mechanism of calcium carbonate in water. Geochim
Cosmochim Acta 51: 2757-2767.
Pach L, Hrabe Z, Komarneni S, Roy R. 1990. Controlled
crystallization of vaterite from viscous solutions of organic colloids. J Mater
Res 5: 2928-2932.
Plummer LN, Busenberg E. 1982. The solubilities of
calcite, aragonite and vaterite in CO2-H2O solutions
between 0 and 90ºC, and an evaluation of the aqueous model form the system CaCO3-CO2-H2O.
Geochim Cosmochim Acta 46: 1011-1040.
Plummer LN, Parkhurst DL, Fleming
GW, Dunkle SA. 1988. PHRQPITZ, a computer program incorporating Pitzer's
equations for calculation of geochemical reactions in brines. US Geological
Survey Water Resources Investigation Report 88-4153. Washington: Goverment
Printing Office.
Prien EL, Frondel C. 1947. Studies in urolithiasis; I.
The composition of urinary calculi. J Urology 57: 949-994.
Ridkosil T, Sejkora J, Ondrus P. 1991.
Monohydrocalcite from polymetallic vein of the Vranice Deposit, near Pribram,
Czechoslovakia. Neues Jb Miner Monat 7: 289-295.
Rivadeneyra MA, Ramos-Cormenzana A, García-Cervigón
A.1985. Etude de l'influence du rapport Mg/Ca sur la formation de carbonate par
des bacteries telluriques. Can J Microbiol 31: 229-231.
Rivadeneyra MA, Delgado G, Delgado R, Ramos-Cormenzana A.
1998. Biomineralization of carbonates by Halomonas eurihalina in solid media with
different salinities: crystal formation sequence. Res Microbiol 149: 277-287.
Saiz-Jimenez C (Ed.) 1995. The deterioration of
monuments. Special Issue, Sci Total Environ 167, 400 pp.
Sánchez-Moral S, Cañaveras JC, Sanz-Rubio E, Hoyos M, Soler V. 1997. Hydrogeochemical
characterization of Tito Bustillo Cave (northern Spain) Proceedings of the 12th
International Congress of Speleology, Neuchâtel, Suisse. Vol 2: 103-108.
Sánchez-Moral S, Soler V, Cañaveras
JC, Sanz-Rubio E, Van Grieken R, Gysels K. 1999. Inorganic deterioration
affecting the Altamira Cave, N Spain: quantitative approach to wall-corrosion
(solutional etching) processes induced by visitors. Sci Total Environ 243/244:
67-84.
Shirling EB, Gottlieb D. 1966. Methods for
characterization of Streptomyces species.
Int J Syst Bacteriol 16:313-340.
Signorelli S, Peroni C, Camaiti M, Fratini F. 1996. The presence of vaterite in bonding mortars of marble inlays from
Florence Cathedral. Mineral Mag 60: 663-665.
Sims SD, Didymus JM, Mann S. 1995. Habit modification
in synthetic crystals of aragonite and vaterite. J Chem Soc Chem Commun:
1031-1032.
Smith KS, Ferry JG. 2000. Prokaryotic carbonic
anhydrases. FEMS Microbiol Rev. 24: 335-366.
Tripp BC, Smith K, Ferry JG. 2001. Carbonic anhydrase:
new insights for an ancient enzyme. J Biol Chem 276: 48615-48618.
Tsuno H, Kagi H, Akagi T. 2000. Effects of trace
lanthanum ion on the stability of vaterite and transformation from vaterite to
calcite in an aquatic system. Goldschmidt 2000 Journal of Conference Abstracts
5(2): 1022-1023.
Vdovic N, Kralj D. 2000. Electrokinetic properties of
spontaneously precipitated calcium carbonate polymorphs: the influence of
organic substances. Colloids Surf 161: 499-505.
Verrecchia
EP, Verrecchia KE, 1994. Needle-fiber calcite: a critical review and a proposed
classification. J Sedim Res A64: 650-664
Figure caption.
Figure 1. Scanning electron
micrographs showing Ca-carbonate precipitates. A) Vaterite hemispherulite showing
fibro-radial internal structure (Acinetobacter
sp., Altamira Cave, grown in liquid medium, scale bar: 5 mm). B) Hollow vaterite hemispherulite (Acinetobacter sp., Altamira Cave, grown in liquid medium, scale
bar: 5 mm). C) Monohydrocalcite spherulites in agar media (Rhodococcus sp., Grotta dei Cervi, scale
bar: 50 mm). D) Spheroidal particle in Altamira cave (scale bar: 5 mm). E) Spheroidal particles associated with needle-fiber aragonite
crystals and beaded filaments. Biofilm from Saint Callixtus Catacombs (scale
bar: 5 mm). F) Detail of spheroidal particle fragments. Saint Callixtus
Catacombs (scale bar: 5 mm).
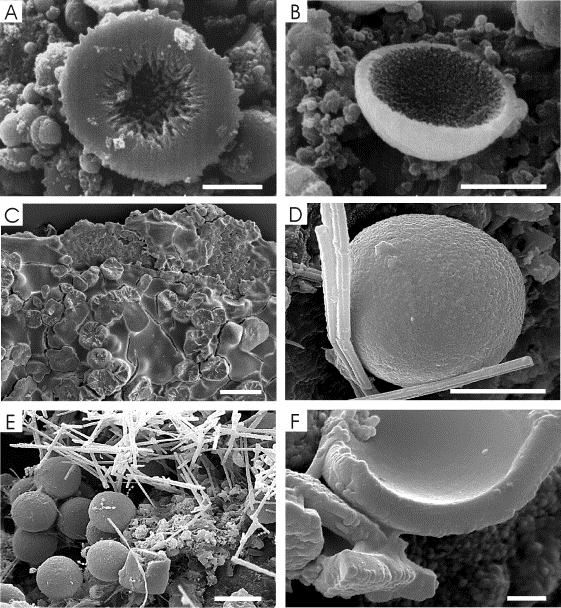
Figure 1